New York, USA, April 29, 2025 (GLOBE NEWSWIRE) -- Phenylketonuria Clinical Trial Pipeline Appears Robust With 25+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight | DelveInsight Phenylketonuria, which is characterized by the absence or deficiency of an enzyme called phenylalanine hydroxylase (PAH), responsible for processing the amino acid phenylalanine. The development of innovative therapies like enzyme replacements, gene therapy, and improved dietary formulations is transforming PKU management, improving patient outcomes, and drawing significant investment. DelveInsight’s ' Phenylketonuria Pipeline Insight 2025 ' report provides comprehensive global coverage of pipeline phenylketonuria therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the phenylketonuria pipeline domain.
Key Takeaways from the Phenylketonuria Pipeline Report DelveInsight’s phenylketonuria pipeline report depicts a robust space with 20+ active players working to develop 25+ pipeline phenylketonuria drugs. Key phenylketonuria companies such as PTC Therapeutics, Otsuka Pharmaceutical, Relief Therapeutics Holding, BioMarin Pharmaceutical, Tessera Therapeutics, Sanofi, NGGT INC ., Alltrna and others are evaluating new phenylketonuria drugs to improve the treatment landscape.

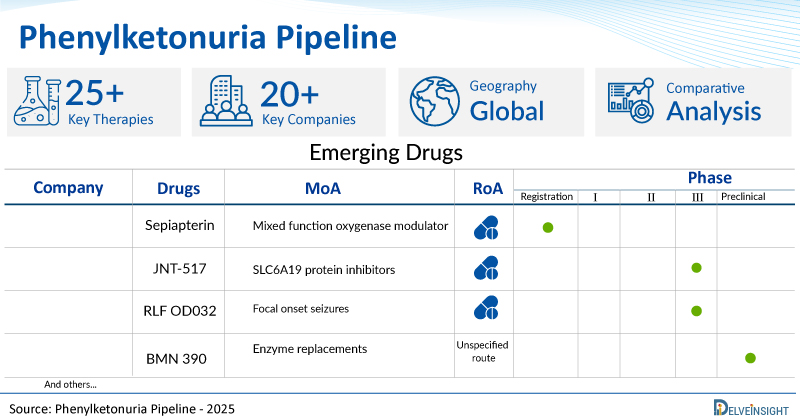
Promising pipeline phenylketonuria therapies such as Sepiapterin, JNT-517, RLF OD032, BMN 390, Gene Writers, SAR444836, NGGT002, AP003, and others are in different phases of phenylketonuria clinical trials. In December 2024, Alltrna announced the presentation of new preclinical data, including of the company's first tRNA development candidate, AP003. AP003 is a chemically modified, engineered tRNA oligonucleotide in a clinically validated, liver-directed lipid nanoparticle (LNP) that can read through the arginine to TGA (Arg-TGA) premature termination codon (PTC).
A single dose of AP003 tRNA showed robust in vivo restoration of protein production to clinically meaningful levels in two transgenic Stop Codon Disease mouse models, methylmalonic acidemia (MMA) and phenylketonuria (PKU). In October 2024, PTC Therapeutics, Inc. announced that the FDA had accepted for filing the New Drug Application (NDA) of sepiapterin for the treatment of pediatric and adult patients living with phenylketonuria (PKU).
A Prescription Drug User Fee Act (PDUFA) target action date is expected to be provided in the Day 74 Letter. In July 2024, PTC Therapeutics, Inc. announced the submission of the sepiapterin NDA to the U.
S. FDA. The NDA submission is for the treatment of pediatric and adult patients with phenylketonuria (PKU), including the full spectrum of ages and disease subtypes.
In September 2024, Jnana Therapeutics released results from the second-dose cohort of 150mg BID (twice daily) from its Phase I/II clinical trial of JNT-517 in adults with phenylketonuria (PKU). In August 2024, Otsuka Pharmaceutical Co., Ltd.
(Otsuka) and Jnana Therapeutics Inc. announced that they had entered into a definitive merger agreement pursuant to which Otsuka will acquire Jnana, making it a wholly owned subsidiary through Otsuka's 100-percent owned subsidiary, Otsuka America, Inc. (OAI).
Request a sample and discover the recent advances in phenylketonuria drugs @ Phenylketonuria Pipeline Report The phenylketonuria pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage phenylketonuria drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the phenylketonuria clinical trial landscape. Phenylketonuria Overview Phenylketonuria (PKU) is a metabolic disorder that disrupts the body’s ability to maintain chemical balance, potentially leading to serious health complications. In PKU, the normal metabolic pathway that converts the amino acid phenylalanine into tyrosine is impaired.
This disruption causes phenylalanine and its byproducts to accumulate in the body, particularly in the brain, leading to intellectual disabilities and skin-related symptoms if untreated. PKU belongs to a group of inherited metabolic diseases known as toxic accumulation inborn errors of metabolism (IEMs), where the buildup of an amino acid or its derivatives becomes harmful. The root cause of PKU is a deficiency in phenylalanine hydroxylase (PAH), the enzyme responsible for converting phenylalanine (Phe) into tyrosine (Tyr).
Tyrosine, while normally a nonessential amino acid, becomes conditionally essential in individuals with PKU. Under typical conditions, phenylalanine—derived from dietary sources and protein breakdown—is converted into tyrosine by PAH with the help of tetrahydrobiopterin (BH4), oxygen, and iron. Additionally, Phe can be converted to phenylethylamine through Phe-decarboxylase.
In PKU patients, the lack of PAH leads to an accumulation of Phe in the blood, reaching toxic levels in the brain. Excess Phe is instead metabolized into compounds like phenylpyruvate, phenylacetate, and phenyllactate, all of which are neurotoxic. Phe also competes with other large neutral amino acids (LNAAs) for transport across the blood-brain barrier via the LAT1 transporter, disrupting the balance of essential amino acids in the brain.
Moreover, reduced levels of tyrosine impair the production of critical neurotransmitters like dopamine, norepinephrine, and epinephrine, contributing further to brain dysfunction. PKU is usually identified soon after birth through newborn screening programs, which often utilize tandem mass spectrometry to measure Phe and Tyr levels in dried blood samples. This method improves accuracy and reduces false results compared to older techniques like the Guthrie test.
Modern screening increasingly relies on automated and quantitative tests, such as fluorimetric assays. Treatment for PKU primarily involves strict dietary management tailored to age, focusing on controlling intake of phenylalanine, tyrosine, protein, and calories. Other approaches include supplementation with large neutral amino acids to reduce Phe transport into the brain, enzyme substitution therapy, and treatment with BH4 (tetrahydrobiopterin), a cofactor that can enhance residual PAH activity.
Sapropterin dihydrochloride (Kuvan), a synthetic form of BH4, has proven effective in some patients with specific PAH mutations. Clinical trials have shown that certain individuals with classical PKU can respond positively to BH4 therapy, depending on the nature of their genetic variant. Find out more about phenylketonuria drugs @ Phenylketonuria Treatment A snapshot of the Pipeline Phenylketonuria Drugs mentioned in the report: Learn more about the emerging phenylketonuria therapies @ Phenylketonuria Clinical Trials Phenylketonuria Therapeutics Assessment The phenylketonuria pipeline report proffers an integral view of the emerging phenylketonuria therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Phenylketonuria Pipeline Report Coverage : Global Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular Therapeutics Assessment By Molecule Type : Monoclonal antibody, Small molecule, Peptide Therapeutics Assessment By Mechanism of Action : Mixed function oxygenase modulators, SLC6A19 protein inhibitors, Enzyme replacements Key Phenylketonuria Companies : PTC Therapeutics, Otsuka Pharmaceutical, Relief Therapeutics Holding, BioMarin Pharmaceutical, Tessera Therapeutics, Sanofi, NGGT INC., Alltrna and others. Key Phenylketonuria Pipeline Therapies : Sepiapterin, JNT-517, RLF OD032, BMN 390, Gene writers, SAR444836, NGGT002, AP003 and others.
Dive deep into rich insights for new phenylketonuria treatments, visit @ Phenylketonuria Drugs Table of Contents For further information on the phenylketonuria pipeline therapeutics, reach out @ Phenylketonuria Therapeutics Related Reports Phenylketonuria Epidemiology Forecast Phenylketonuria Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted phenylketonuria epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Phenylketonuria Market Phenylketonuria Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key phenylketonuria companies, including PTC Therapeutics, Synlogic, among others. Alzheimer's Disease Market Alzheimer's Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Alzheimer's disease companies, including BioVie, AB Science, Cassava Sciences, TauRx Therapeutics, Novo Nordisk, KeifeRx, Eli Lilly, AriBio, Cerecin, Alzheon, Neurim Pharmaceuticals, Syneos Health, Athira Pharma, Annovis Bio, Anavex Life Sciences, AgeneBio, Eisai, among others. Alzheimer's Disease Pipeline Alzheimer's Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Alzheimer's Disease companies, including Biogen, AZTherapies, Cerecin, Neurotrope, Synaptogenix, INmune Bio, Cassava Sciences, EIP Pharma, Neuraly, AB Science, Cortexyme, Anavex Life Sciences, Athira Pharma, Time Therapeutics, Denali Therapeutics Inc.
, Alector Inc., Lexeo Therapeutics, TrueBinding, Inc., Vaccinex Inc.
, Annovis Bio Inc., Eisai Inc., Hoffmann-La Roche, Ionis Pharmaceuticals, Inc.
, Otsuka Pharmaceutical Co., Ltd., Cognition Therapeutics, Merck Sharp & Dohme LLC, ImmunoBrain Checkpoint, AbbVie, AriBio Co.
, Ltd., Oryzon Genomics S.A.
, Eli Lilly and Company, Neurokine Therapeutics, Excelsior, Seelos Therapeutics, Inc., Janssen Research & Development, LLC, Shanghai Hengrui Pharmaceutical Co., Ltd.
, reMYND, Alzinova AB, VTBIO Co. LTD, BioVie Inc., Prothena Corporation plc, Coya Therapeutics, Inc.
, among others. Psychosis in Parkinson’s and Alzheimer’s Disease Market Psychosis in Parkinson’s and Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key psychosis in Parkinson’s and Alzheimer’s disease companies, including Sunovion Pharmaceuticals, Karuna Therapeutics, Vanda Pharmaceuticals, Suven Life Sciences, Enterin, Intra-Cellular Therapies, Merck Sharp & Dohme, among others. DelveInsight’s Pharma Competitive Intelligence Service : Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies.
These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion. Other Business Consulting Services Healthcare Conference Coverage Pipeline Assessment Healthcare Licensing Services Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study About DelveInsight DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. Connect with us at LinkedIn.
Phenylketonuria Clinical Trial Pipeline Appears Robust With 25+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight | DelveInsight
Phenylketonuria, which is characterized by the absence or deficiency of an enzyme called phenylalanine hydroxylase (PAH), responsible for processing the amino acid phenylalanine. The development of innovative therapies like enzyme replacements, gene therapy, and improved dietary formulations is transforming PKU management, improving patient outcomes, and drawing significant investment. Phenylketonuria, which is characterized by the absence or deficiency of an enzyme called phenylalanine hydroxylase (PAH), responsible for processing the amino acid phenylalanine. The development of innovative therapies like enzyme replacements, gene therapy, and improved dietary formulations is transforming PKU management, improving patient outcomes, and drawing significant investment.